Cancers of lung, breast, colon, and prostate account for approximately 60-70% of all cancers occurring annually. With the CE-IVD certification of all four liquid biopsy assays for exactly these types of cancers, 4D Lifetec is now in a position to provide the vast majority of cancer patients in Europe with this much-needed help in early detection.
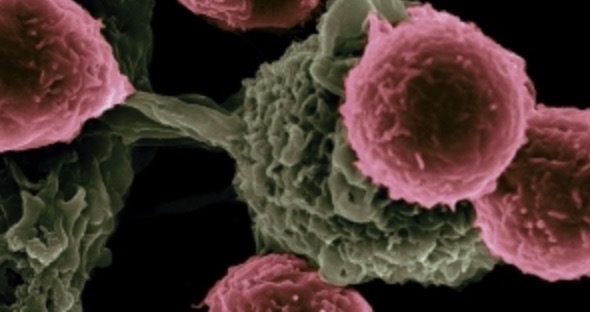
A new liquid biopsy platform for cancer detection analysing patient derived peripheral blood mononuclear cells (PBMCs)
4D Lifetec offers 4D Lifetest™, the first diagnostic method that provides detection of multiple cancer types at the earliest possible stage; in fact, the assay shows the same sensitivity for stage 1 cancers as it does for stage 4. 4D Lifetest™ requires only a small volume of blood, and has significantly lower cost and shorter time than other liquid biopsy tests. The method is being clinically validated and shows dramatically better performance than current diagnostic methods including the genomic assays developed by Grail and Thrive. Offering the first high sensitivity, non-genomic based liquid biopsy, 4D Lifetest™ enables detection of any cancer type at an early stage.
Source: 4D Lifetec
11-24-2021